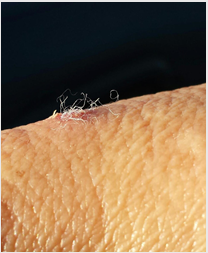
 lymedisease.org
lymedisease.org
MAYO CLINIC:
Morgellons disease: Managing an unexplained skin condition
Morgellons disease is a controversial, unexplained skin condition. Here you’ll find answers to common questions about Morgellons and suggestions for coping with it.
Morgellons disease is an uncommon, poorly understood condition characterized by small fibers or other particles emerging from skin sores. People with this condition often report feeling as if something were crawling on or stinging their skin.
Some doctors recognize the condition as a delusional infestation and treat it with cognitive behavioral therapy, antidepressants, antipsychotic drugs and counseling. Others think the symptoms are related to an infectious process in skin cells. Further study is needed.
Signs and symptoms
People who have Morgellons disease report the following signs and symptoms:
- Skin rashes or sores that can cause intense itching
- Crawling sensations on and under the skin, often compared to insects moving, stinging or biting
- Fibers, threads or black stringy material in and on the skin
- Fatigue
- Difficulty concentrating
- Short-term memory loss
- Depressed mood
The intense itching and open sores associated with Morgellons disease can severely interfere with a person’s quality of life.
What do researchers know about Morgellons disease?
The research on Morgellons by multiple groups over decades has yielded conflicting results. Multiple studies report a possible link between Morgellons and infection with Borrelia spirochetes.
These results contradict an earlier study by the Centers for Disease Control and Prevention (CDC), which concluded that the condition isn’t caused by an infection or parasites. The CDC study of 115 people with Morgellons, which the CDC refers to as an unexplained dermatopathy, showed that most of the fibers in the skin wounds were cotton. The CDC report noted that the condition is most often seen in middle-aged white women, and its symptoms are very similar to those of a mental illness involving false beliefs about infestation by parasites (delusional infestation).
Small research studies have tried to determine the cause and effective treatment for Morgellons disease. But there is still no proven guidance on diagnosis and treatment. Further research is needed.
A contested diagnosis
Common attitudes of health professionals toward Morgellons disease include:
- Thinking that Morgellons disease is a specific condition that needs to be confirmed by research
- Thinking that signs and symptoms of Morgellons disease are caused by another condition, often mental illness
- Not acknowledging Morgellons disease or reserving judgment until more is known about it
Some people who suspect they have Morgellons disease claim they’ve been ignored or dismissed as fakers. In contrast, some doctors say that people who report signs and symptoms of Morgellons disease typically resist other explanations for their condition.
Coping with Morgellons disease
The signs and symptoms linked to Morgellons disease can be distressing. Even though health professionals may disagree about the nature of the condition, you deserve compassionate treatment. To manage your signs and symptoms:
- Establish a relationship with a caring health care team. Find a doctor who acknowledges your concerns, does a thorough examination, talks through treatment options with you and works with a multidisciplinary team.
- Be patient. Your doctor will likely look for known conditions that point to evidence-based treatments before considering a diagnosis of Morgellons disease.
- Keep an open mind. Consider various causes for your signs and symptoms and discuss your doctor’s recommendations for treatment — which may include long-term mental health therapy.
- Seek treatment for other conditions. Get treatment for anxiety, depression or any other condition that affects your thinking, moods or behavior.
History of Morgellons disese: Er dette er reell/ virkelig sykdom?
Clinical, Cosmetic and Investigational Dermatology Dovepress
submit your manuscript | www.dovepress.com
Dovepress
71
REVIEW
open access to scientific and medical research
Open Access Full Text Article
http://dx.doi.org/10.2147/CCID.S152343
History of Morgellons disese: a from delusion
to definition
Marianne J Middelveen1
Melissa C Fesler2
Raphael B Stricker2
1
Atkins Veterinary Services, Calgary,
AB, Canada; 2
Union Square Medical
Associates, San Francisco, CA, USA
Abstract: Morgellons disease (MD) is a skin condition characterized by the presence of
multicolored filaments that lie under, are embedded in, or project from skin. Although the
condition may have a longer history, disease matching the above description was first reported
in the US in 2002. Since that time, the condition that we know as MD has become a polemic
topic. Because individuals afflicted with the disease may have crawling or stinging sensations
and sometimes believe they have an insect or parasite infestation, most medical practitioners
consider MD a purely delusional disorder. Clinical studies supporting the hypothesis that MD
is exclusively delusional in origin have considerable methodological flaws and often neglect
the fact that mental disorders can result from underlying somatic illness. In contrast, rigorous
experimental investigations show that this skin affliction results from a physiological response to
the presence of an infectious agent. Recent studies from that point of view show an association
between MD and spirochetal infection in humans, cattle, and dogs. These investigations have
determined that the cutaneous filaments are not implanted textile fibers, but are composed of
the cellular proteins keratin and collagen and result from overproduction of these filaments in
response to spirochetal infection. Further studies of the genetics, pathogenesis, and treatment
of MD are warranted.
Keywords: Morgellons disease, dermopathy, Lyme disease, Borrelia burgdorferi, spirochetes
Introduction
Morgellons disease (MD) is a disfiguring and perplexing skin condition associated with
spirochetal infection and tick-borne illness.1–7 This poorly understood condition has a
worldwide distribution, with estimated self-reported cases numbering over 14,000 in
2009.5
Since that time, there has been an increasing number of individuals reported
to be afflicted with this disorder (C Casey, Charles E Holman Morgellons Disease
Foundation, personal communication, 2017). The distinguishing diagnostic feature of
MD is spontaneously appearing ulcerative skin lesions that contain unusual filaments
lying under, embedded in, or projecting from the skin. The characteristic filaments
are microscopic, visually resembling textile fibers, and are white, black, or a more
vibrant color, such as red or blue.1–7 In addition to fiber production, some patients may
experience formication, described as stinging, biting, creeping and crawling sensations.
The symptoms of MD are not limited to the skin. MD patients experience a variety
of systemic manifestations, such as fatigue, joint pain, cardiac complications, cognitive difficulties, and neuropathy, all symptoms that are commonly reported by Lyme
disease (LD) patients.1–7
Correspondence: Raphael B Stricker
Union Square Medical Associates,
450 Sutter Street – suite 1504,
San Francisco, CA 94108, USA
Tel +1 415 399 1035
Email rstricker@usmamed.com
Journal name: Clinical, Cosmetic and Investigational Dermatology
Article Designation: REVIEW
Year: 2018
Volume: 11
Running head verso: Middelveen et al
Running head recto: Morgellons disease
DOI: http://dx.doi.org/10.2147/CCID.S152343
Video abstract
Point your SmartPhone at the code above. If you have a
QR code reader the video abstract will appear. Or use:
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
72
Middelveen et al
History
The name “Morgellons” (pronounced with either a hard or
soft “g”) comes from a letter written in 1674 by Sir Thomas
Browne, an English physician. The letter contains a brief
description of a skin disease in French children:
Hairs which have most amused me have not been in the
face or head, but on the back, and not in men but children,
as I long ago observed in that endemial distemper of little
children in Languedock, called the Morgellons, wherein
they critically break out with harsh hairs on their backs,
which takes off the unquiet symptoms of the disease, and
delivers them from coughs and convulsions.8
Browne’s description of “the Morgellons” and other historical accounts of similar maladies date from 1544–1884 and
were found in Browne’s library in 1935 by Kellett, who then
summarized and discussed them.8
The accounts by Browne and others were likely referring
to a heterogeneous group of skin conditions that may have
differed from the skin condition that we refer to as MD today.
These early accounts describe primarily childhood illnesses,
many of which were associated with convulsions. There is
mention of hairs, worms (with black protruding heads), or
comedones that protruded from the skin, primarily on the
arms, legs, and back, and at that time there was much debate
as to whether these objects were animate or inanimate.8
Ettmüller, for example, provided a drawing of infesting
organisms that look like various arthropods, some resembling scabies mites, while the famous Dutch microscopist
Leuvenhoeck concluded that such objects were inanimate.8
In 1894, Thibierge described patients who had erroneous
and unshakeable beliefs of skin infestation by parasites, and
proposed the name “acarophobia”.9,10 In 1946, Wilson and
Miller suggested that “acarophobia” should be replaced by
the name “delusions of parasitosis” (DOP).10,11
From 1902 to 1938, case studies describing “parasitophobias” or “dermatological hypochondriasis” that resulted in
delusional interpretation of skin sensations were published
sporadically.12–20 However, as early as 1935, an association
between spirochetal infection and DOP was documented by
the French physician Vié, who reported that six of eight of the
subjects in his case studies had syphilis.18 In 1938, a pivotal
narrative of DOP was published by Ekbom, a series of case
studies describing patients who had sensations of movement
and the belief that insects were crawling on or under skin.
Ekbom felt that determining the underlying cause of the
formication was important, stating that “it is the underlying
illness that determines the overall presentation of the beliefs”
and “it is perhaps too simple that the parasitophobias should
be considered as mental illness and nothing more”.20 Interestingly, like Vié, Ekbom found that spirochetal infection was
present in his patient cohort, and three of Ekbom’s seven
patients had documented cases of syphilis. Despite the fact
that syphilis was considered rare in Sweden, Ekbom did not
believe that spirochetal infection was a contributing factor.20
Ekbom reported that the skin sensations consisted mostly
of itching, but also that there was a feeling that something
was crawling on or under the skin, and that stabbing and biting sensations could also occur. He mentioned that in such
cases, “little animal” specimens were sometimes brought in
by patients to show to physicians and that such collections
consisted of “little hairs, little threads, grains of sand, and
skin scales”. He noted that apart from delusional ideas of
infestation, no consistent mental problems were present.20
Although Ekbom could not find any arthropods, parasites,
or other microscopic animals, it is important to note that he
found hairs, “little threads”, and “grains of sand” in patient
specimens. His description is consistent with the findings of
unusual hairs, fibers, and hardened comedo-like dermatological objects that we see in MD specimens.20 Such objects will
be discussed in depth later in this report.
It is possible that patients in the case studies written by
other physicians and mentioned by Ekbom had syphilis or
other spirochetal infections. The causative agent of syphilis
was first reported in 1905 by Fritz Schaudinn and Erich
Hoffmann, who used dark-field microscopy and described
spiral-shaped bacteria – Spirochaeta pallida – now called
Treponema pallidum.21 The first test for syphilis was developed shortly afterward in 1906 by German physician and
bacteriologist August von Wassermann. The Wassermann
test was a complement-fixation test that detected antibodies
reactive to the syphilis spirochete. The Wassermann tests
performed in the 1920s and 1930s lacked accuracy,22,23 and
cases of syphilis among patients with delusional parasitosis
(DP) may have gone unacknowledged as a result.
Regardless of the test accuracy for syphilis, it is possible
that some of the patients described in these historical case
studies may have been infected with Borrelia spp., other
treponemes or Leptospira spp. B. burgdorferi (Bb) is not
a new organism: the earliest known case dates back 5,300
years in the mummy dubbed Ötzi,24 and Borrelia DNA was
also detected in two museum specimens of the white-footed
mouse, Peromyscus leucopus, collected in 1894.25 Spirochetes resembling Borrelia have also been found in amberfossilized ticks from 15–20 million years ago.26 Therefore,
spirochetal infections associated with MD may have occurred
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
73
Morgellons disease
periodically hundreds or even thousands of years ago in
human history, yet have gone unrecognized and unreported.
There is a brief mention of “the Morgellons” by EmslieSmith in 1946, where he proposes that the condition was a
form of myiasis caused by the larva of a Hypoderma species,
although his account did not provide convincing evidence
to support his theory.27 In a 1983 lecture, Lyell described a
survey of several hundred dermatologists treating patients
with DOP who reported that many of their patients exhibited
specimens in matchboxes, baggies, scraps of paper, or photographs. Lyell labeled this practice the “matchbox sign”.28 The
survey was reported in a short editorial in the Lancet,
10 after
which the “matchbox sign” was adopted by dermatologists
as being proof of delusional mental illness.29–31 Likewise, the
manipulation of skin to extract specimens for relief was also
considered to be proof of having a delusional disorder, and
this practice was labeled “the tweezer sign”.29
After Emslie-Smith’s mention of MD in 1946, there
were no significant references to MD in medical literature
until 2002. In 2001, biologist Mary Leitao noted nonhealing lesions on her young son, who complained that he had
“bugs” under his skin. She removed a scab, and upon magnification she did not see arthropods or parasites, but she
did see embedded blue and red filaments. Leitao searched
the Internet looking for similar conditions, and Browne’s
description bore a resemblance to her son’s condition, so
she appropriated the name.1,2 Leitao subsequently founded
the not-for-profit Morgellons Research Foundation (MRF).
The MRF website included a database where those with the
disorder could self-report their skin and systemic symptoms.5
Leitao did not get answers from the mainstream medical establishment. She had sought help from many doctors,
including Fred Heldrich, a Johns Hopkins pediatrician, who
arrived at the conclusion that Leitao should not use her son
to “explore the problem” and that she could benefit from a
psychiatric evaluation.32 Leitao gathered a group of patient
advocates, medical practitioners, physicians, and nurses into
a volunteer board of directors, which included Georgia-based
pediatrician Greg Smith, Texas-based nurse practitioner
Virginia Savely, patient advocates Charles E Holman and
Cindy Casey-Holman, and former National Aeronautics
and Space Administration (NASA) physician and researcher
William Harvey32 (C Casey, Charles E Holman Morgellons
Disease Foundation, personal communication 2017). Leitao
also sought help from Randy Wymore, a pharmacology professor at Oklahoma State University.32
In 2006, Dan Rutz, a spokesman for the US Centers for
Disease Control and Prevention (CDC) contacted Leitao
and said that the CDC would form a task force to investigate MD, declaring that “these people deserve more than to
be blown off ”.32 The CDC published their study results in
2012, declaring that MD was “similar to more commonly
recognized conditions, such as delusional infestation [DI]”.33
As of 2012, Leitao had withdrawn from the public eye and
closed the MRF. The website run by the MRF is no longer
active, and the domain name was taken over by others, now
promoting fringe etiologic theories of MD.
Diagnosing MD
In light of previous studies of MD,1–7 a case definition for MD
is proposed: a somatic LD-like illness associated with spontaneously appearing, slowly healing, filamentous, ulcerative
skin lesions, with the key diagnostic criterion being colored,
white, or black filaments protruding from or embedded in
skin. Patients diagnosed with MD, either by self-diagnosis or
by a health care practitioner, are not a homogeneous group,
thus highlighting the need for a universally accepted case
definition.
Filaments in MD lesions usually require magnification
of 50× or more to be seen, and at that magnification they can
be mistaken for textile fibers. Health care providers need to
be objective when viewing these fibers: a patient must have
unusual filaments visible under 50× magnification or higher
(as opposed to the magnification of 10× normally used in
dermatology) and embedded in or extruding from skin to
be diagnosed with MD. The filaments are relatively easy
to see with proper visualization tools, and detectable fibers
should not be automatically dismissed as “self-implanted”
or composed of synthetic substances without an appropriate
evaluation. Mental health status is not a diagnostic factor in
MD cases, as outlined herein.
Controversy
Unlike Ekbom, who was concerned about the underlying
cause of DP,8
many modern-day practitioners and scientists
have ignored the potential underlying causes responsible for
formication and beliefs of infestation. It is easier to declare
mental illness the exclusive etiologic cause, thus blaming the
patient, when confronted with perplexing symptoms that the
practitioner cannot explain. However, it is irresponsible to
label a patient delusional without an appropriate psychiatric
evaluation, and if mental illness is present a physician should
bear in mind that an underlying infectious process can cause
a pathological response resulting in mental illness.
A PubMed search using the keyword “Morgellons”
yielded 58 articles, the earliest dating from 1946. From 2006
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
74
Middelveen et al
to present, medical literature is divided into two polarized
points of view. One point of view is that MD is a form of
delusional mental illness, and the other is that underlying
spirochetal infection causes a filamentous dermopathy that is
accompanied by an array of LD-like multisystem symptoms
that may or may not include neuropsychiatric symptoms.
There are approximately 40 papers in the medical literature
proposing that MD is purely a delusional disorder, and only
a quarter of that figure proposing that MD has an infectious
etiology.
Diagnosing delusional disorder
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM)-V makes no mention of a diagnosis of DOP. The closest diagnosis is Delusional
disorder 297.1 (F22), somatic type, which is defined thus:
• presence of one or more delusions with a duration of
one month or longer
• criteria for schizophrenia have never been met (note
hallucinations if present are not prominent and are
related to the delusional theme eg, the sensation of
being infected with insects is associated with delusions
of infestation)
• apart from the impact of the delusion(s) or its ramifications, functioning is not markedly impaired, and
behavior is not obviously bizarre or odd
• if manic or major depressive episodes have occurred,
these have been brief relative to the duration of the
delusional periods
• the disturbance is not better explained by another mental
disorder, such as obsessive compulsive disorder, and is
not due to the physiological effects of a substance or
medication or another medical condition.34
Somatic-type delusional disorders manifest with core beliefs
concerning bodily functions or sensations. Manschreck
stated that the diagnosis of delusional disorder should be a
diagnosis of exclusion, and he outlined three steps for evaluating patients with delusions. The first step is to establish if
pathology is present. This step requires clinical judgment to
distinguish among a true observation, a firm belief, an overvalued idea, and a delusion.35 He states that a comment that
at first appears delusional can prove to be factual, and some
reports that seem believable may later be found to be delusional. Therefore, he recommended that rather than the truth
or falseness of a belief, the extremeness or inappropriateness
of a patient’s behavior may be the determining factor leading
to a diagnosis of delusional disorder.35,36 In other words, one
must first establish that a belief is delusional, and not the
result of an underlying somatic illness.
The second step involves determining if characteristics
associated with delusions, such as confusion, agitation, perceptual disturbances, physical symptoms, and mood abnormalities are present.35 The third step is performing a systematic
differential diagnosis, including a thorough history, mental
status examination, and laboratory/radiological evaluation to
rule out other medical and psychiatric conditions that present
with delusions.35 The status examination, including cognitive
status, is usually normal, except for the delusional beliefs:
memory and cognition are intact.37 Auditory or visual hallucinations are indicative of more severe psychotic disorders,
such as schizophrenia, and suggest exclusion of a delusional
disorder.37 The differential diagnosis should exclude medical
conditions that can cause delusions, such as neurodegenerative or other central nervous system disorders, vascular
diseases, vitamin deficiencies, medications, metabolic and
endocrine disorders, toxins, and infectious diseases.37 Note
that spirochetal infections such as syphilis and LD could cause
symptoms that fall into this category.
Evidence supporting the hypothesis
of DP
Among reports that promote a delusional etiology for MD,
there are a number of review articles, opinion pieces, or editorial letters.29,30,38–53 These do not provide any new research or
clinical evidence to support the claims that MD is a delusional
disorder, but they do present common discussion themes
that are frequently reiterated in case studies and research
papers.54–76 Common discussion themes are:
• MD is a delusional disorder29,30,38–76
• MD is a variation of DOP, DP, or DI29,30,38–76
• MD is defined as the fixed, unshakable belief, despite lack
of medical evidence, of being infested with microscopic
organisms or inanimate objects29,30,38–76
• the presentation of specimens in or out of a container,
whether it be a matchbox, small plastic bag, paper, pill
container, or photographic image, etc, is diagnostic of
DOP, DP, or DI29–31,52,55,59–62,64,66,70,71
• patients with MD tend to have psychiatric comorbidities74,68
• MD is a mass delusional mental illness afflicting primarily
middle-aged Caucasian females68,69,74,76
• delusions of infestation are spread from person to person
and transmitted by the Internet30,39,41,51,52,54,58,60
• antipsychotic drugs are the treatment of choice for
MD29,30,38–62,64–76
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
75
Morgellons disease
• electroconvulsive therapy is an acceptable treatment for
MD30,52
• establishing rapport to gain confidence and trust helps
convince patients to take antipsychotic drugs30,39,41,49,54,58,60
• using the word “Morgellons” in dialogue with patients
can help establish rapport and trust30,39,41,54,58,60
• it is acceptable for dermatologists to diagnose delusional
disorder and prescribe antipsychotic medication51,56
• use of deceptive dialogue and strategies aimed at convincing patients to take antipsychotic drugs is a justifiable
practice41,50,54,60,65
• if a patient’s friend(s) or family member(s) also observe a
subject’s dermatological lesions and believe the evidence,
then they too are considered to share the delusional belief,
and the delusion is considered to have been transmitted
from one individual to another;30,51,54,68 the belief shared
by two people that there are organisms present in the skin
is called folie à deux (madness of two); folie à trois, folie
à quatre, or folie à cinq are shared beliefs by three, four,
or five people, respectively; and shared belief in a family
is folie à famille.30,51,54,68
A PubMed search using the keyword “Morgellons” identified 18 publications consisting of case studies of between
one and six patients. Table 1 provides a summary of the case
studies. Most of these patients clearly do not meet the case
definition of MD. Case studies provide useful anecdotal
evidence, but they have limitations. Of these case studies,
the majority do not mention the observation of fibers being
present in or projecting from the skin (the key defining criterion for MD), nor do they mention whether the attending
health care professional looked for filaments in the skin at
magnification of 50× or higher.54–56,59–68,70,73
Some studies reported that patients presented specimens
to the health care provider as evidence – fibers, lint, hair and
skin scrapings, etc – and this was interpreted as being diagnostic of DI.55,59–62,64,66,70,71 In many of these case studies, there
was no mention of any analysis of patient-supplied specimens
to determine composition.54,59,60 In a few cases, the health care
provider did nothing more than a gross visual identification
of patient-provided specimens.55,62,66,70,71 Some studies did not
mention fibers associated with their cases at all.55,58,60 Only
seven of these studies indicated that the subjects had heard
of MD.54,56,58,61–63,70
In some case studies, a patient was diagnosed with DP
or DI on very little evidence. Bhandary et al diagnosed DP
in patients who felt crawling sensations and thus thought
they had bugs in their ears or nose.55 Such conditions as
seborrheic dermatitis or eczema can cause formication and
crawling sensations inside the ears and nose, and should
be ruled out before diagnosing mental illness. Sandhu and
Steele diagnosed a patient with DI because the patient felt
as though she had fibers growing into her eye. The patient
had ectropion, and perhaps this was a factor contributing to
the uncomfortable sensations.73 In these cases, underlying
causes for sensations were not thoroughly investigated before
assuming the patient was having sensory hallucinations.
Furthermore, given the sensations of formication, the beliefs
in bugs in the first cases and of fibers in the second case are
not unreasonable or inappropriate.
In cases where a health care professional did not look
for filaments, it is unclear whether or not patients with MD
were in these studies. Some studies did not mention if the
patient had lesions.54 Some reports mentioned lesions or
skin abnormalities, but did not describe examination with
magnification of 50× or higher for fibers in or projecting
from skin.56,58–60,62–67,70,73 Other studies mentioned that the skin
was completely normal and that no skin abnormalities were
present; therefore, it is very unlikely that patients in these
studies actually had MD, as they did not have the diagnostic
clinical finding.55,61,65,71,75
There are only three case studies that specifically mention
the presence of fibers either projecting from or embedded in
skin.57,72,75 Roncati et al72 reported “grayish spots” under skin,
then used scanning electron microscopy (SEM) and energydispersive spectroscopy (EDS) to study the spots. SEM showed
that the spots were associated with fibers described as “synthetic
wire” consistent with samples from a washing machine, as well
as keratin fibers consistent with hair from the patient’s dog.
The EDS analysis detected carbon, sulfur, and oxygen peaks
– elements for keratin – but EDS could not reveal what type of
keratin it was. Therefore, the “synthetic wire” could have been
keratin filaments from the patient. The authors concluded that
the keratin hairs were of canine origin, based on morphological resemblance to the patient’s dog hair, yet they provided no
further proof of this conclusion. As some MD fibers are small
hairs, determining whether the hairs are human or canine in
origin is important. SEM shows only the outer shape of a specimen, so the scaling pattern is the only morphological feature
available for comparison, and the imbricate scaling of canine
and human hair is quite similar (TA Evans, TRI Princeton,
personal communication, September 13, 2017). EDS analysis
can only tell the chemical composition of the specimen and
again, human and canine hair would be similar.77
Ohn et al saw only one black fiber protruding from the
skin, and then claimed that this single fiber was lost during
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
76
Middelveen et al Table 1 Case studies claiming delusional etiology of Morgellons disease Study Case Complaint Fibers present on skin Fibers studied Detection of spirochetes Underlying conditions Ohn et al75 30-year-old female Fibers under skin, stinging in fingers One fiber on dermal side of
biopsy
Lost during processing Yes, but different methods
in abstract and text
indicated negative results
Not reported
Ranka et al71 50-year-old
female
Fibers coming out of skin Not reported Patient supplied specimen,
visual identification
Not reported Not reported
Roncati et al72 49-year-old
female
Fibers coming out of skin Yes, gray spots
containing fibers
Yes, used SEM/EDS,
methods not sufficient
to determine source of
keratin fibers
Yes, used serology, but
did not indicate if positive
or negative, methodology
insufficient
HCV infection, “myoclonies” [sic]
Sandhu and
Steele73
65-year-old
female
Sensations of fibers in eyelid, “wormy
things” coming from skin, self diagnosed
with MD
Not reported Not reported Not reported Ectropion
Mortillaro et al70 37-year-old
female
“Black substance” coming out of hands Not reported Not reported Not reported Amphetamine and buprenorphine use
Fellner67
Case 1
44-year-old
female
Crawling sensations, strings and
parasites coming out of eyelids,
eyelashes, and ears
Not reported Not reported Not reported History of cocaine and heroin use,
seborrheic dermatitis
Fellner67
Case 2
90-year-old
female
Worms and strings coming out of body Not reported Not reported Not reported Senile dementia, Trichuris infection
Altunay et al66
Case 1
65-year-old
female
Pruritis, stinging on head, paper and
wood emerging from skin
Not reported Looked at patient-supplied
specimen
Not reported Vitamin B12 deficiency, cranial trauma
confirmed by MRI, hypertension
Altunay et al66
Case 2
71-year-old
female
Pruritis, sand-like material out of skin,
belief of infection
Not reported Indicated specimens were
presented, but did not
indicate if they looked at
them
Not reported Vitamin B12 deficiency, psychosis not
otherwise specified
Altunay et al66
Case 3
53-year-old
female
Burning sensations, worms coming out
of skin
Not reported Not reported Not reported Vitamin B12 deficiency,
hypercholesterolemia, hyperglycemia,
hypothyroidism, cultural beliefs may have
been a factor
Altunay et al66
Case 4
27-year-old
female
Bugs crawling under scalp and hair loss Not reported Not reported Not reported Vitamin B12 deficiency, schizoaffective
disorder
Altunay et al66
Case 5
65-year-old
female
Pruritus and bugs on head Not reported Looked at dandruff flakes
for lice
Not reported Brief psychotic disorder, vitamin B12
deficiency
Altunay et al66
Case 6
65-year-old
female
Pruritus and crawling sensations,
believed a snake migrated in her body
Not reported Not reported Not reported Schizophrenia
Dewan et al62
Case 1
68-year-old
female
Cutaneous erosions and hair loss,
thought a Mahonia bush was inside her
Not reported Patient brought specimen,
described as normal,
methodology not specified
Not reported Reactive depression
Dewan et al62
Case 2
63-year-old
male
Fibers in scalp, eyebrows, and ears Not reported Not reported Previous infestation with lice, used LSD
and other hallucinogens
(Continued)
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
77
Morgellons disease
Study Case Complaint Fibers present
on skin
Fibers studied Detection of
spirochetes
Underlying conditions
Grosskopf et al64 39-year-old
woman
Insect and hair emerging from maxillary
gingiva
Not reported Patient supplied samples
from feet, visually identified
as hair
Not reported Bipolar disorder, admitted rubbing gingiva
with cloth
DeBonis and
Pierre61
46-year-old
male
Pursued by Japanese mafia, crawling
sensations, cocoons emerging from
body, self-diagnosed with MD
Not reported Not reported Not reported Used cannabinoids, ivermectin toxicity
Robles et al43
Case 1
60-year-old
male
Reported hard hair bulbs, and that
cartilage and fibrous material emerged
from skin
Not reported Not reported Not reported Secondary bacterial infection, treated
successfully with doxycycline
Robles et al43
Case 2
35-year-old
female
Reported black fibers in skin, denied
parasites or organisms
Not reported Not reported Not reported Secondary bacterial infection, treated
successfully with doxycycline
Robles et al43
Case 3
50-year-old
female
Reported rocks and fibrous threads
in skin
Not reported Not reported Not reported Not reported
Freudenreich
et al58
43-year-old
male
Believed he had parasites, selfdiagnosed with MD Not reported Not reported Not reported HIV infection
Reid and Lio60 47-year-old
female
Painful lesions with thread-like fibers Not reported Brought in bloody
specimens on paper towels,
did not indicate they were
studied
Not reported Sepsis, diabetes, restless-leg syndrome,
fibromyalgia
Dovigi57 61-year-old
female
Reported fibers coming out of oral
lesion
Yes, three fibers
removed
SEM showed little
microstructure and
homogeneous cross section;
spectrum profile showed
primarily carbon and small
amount of oxygen, consistent
with synthetic fiber
Not reported Hyperthyroidism, osteoporosis, history of
mononucleosis and hepatitis
Harth et al59 55-year-old
female
Fibers and barbs in skin, fatigue,
cognitive dysfunction, night sweats,
sinus pressure
Not reported Brought in skin specimens,
did not indicate they were
studied
Not reported Not reported
Vila-Rodriguez
and Macewan56
57-year-old
male
Erythematous lesions, self-diagnosed
with MD
Not reported Not reported Not reported Not reported
Bhandary et al55
Case 1
23-year-old
female
Reported a bug in ear Not reported Not reported Not reported Not reported
Bhandary et al55
Case 2
70-year-old
male
Complained of a bug in ear Not reported Not reported Not reported Not reported
Bhandary et al55
Case 3
58-year-old
male
Complained of a bug emerging from
nose, crawling and itching sensations
on nose
Not reported Not reported Not reported Not reported
Murase et al54 45-year-old
female
Self-diagnosed with MD Not reported Not reported Not reported Not reported
Abbreviations: HCV, hepatitis C virus; SEM/EDS, scanning electron microscopy/energy-dispersive spectroscopy; MD, Morgellons disease; MRI, magnetic resonance imaging.
Table 1 (Continued)
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
78
Middelveen et al
processing for histological examination.75 Therefore, one cannot draw any conclusions about the composition or origin of
this fiber. Dovigi provided convincing evidence that the fibers
extracted from an oral lesion on the mucosal distal tuberosity of a tooth were synthetic carbon-based fibers, but there
is no evidence that the fibers were self-implanted.57 Dental
floss is composed of synthetic fibers, such as nylon.78 Fibers
could have been introduced during flossing, especially if the
floss had frayed and become lodged between teeth, eventually festering and causing a lesion. Belief that the fibers had
originated in the tissue would be reasonable under those
circumstances.
The case studies varied in terms of looking for pathogens,
but on further examination all of the studies fall short in
looking for spirochetal infection. Only two of the case studies
looked for Borrelia infection, and neither of these performed
a thorough laboratory analysis to search for spirochetes.72,75
The description of testing for Borrelia in these studies is not
sufficiently detailed to know what was actually done. In science, reproducibility is important, and methodologies should
provide enough details that others can repeat them. Roncati et
al stated: “In adjunct, the patient had noticed an increase in
the viscosity of mucus, saliva, and tears, as to produce four
unexplainable corneal ulcers in the last two years, without a
rise in the autoimmunity or Borrelia spp. serology”.72 There
is no mention of whether or not the Borrelia spp. serology
was interpreted as being positive or negative, what species
or type of Borrelia antigens were used, what laboratory the
serology was performed at, or what method was used to detect
the antibodies. Furthermore, although the patient had a few
gray spots containing fibers, there was no evidence they were
self-implanted or related to a delusional belief. Therefore, the
study cannot disprove an infectious etiology for MD.
The study by Ohn et al is no better in terms of providing
methodology.75 In fact, the methodology in the abstract does
not match the text. While the abstract states that polymerase
chain reaction (PCR) testing for Bb was negative in serum,
the text states that Bb serology was negative. It is thus unclear
whether serology or PCR testing was used to diagnose Bb.75
There is mention that a Warthin–Starry stain was performed,
and histological examination revealed only a mild lymphocytic infiltration. However, because Bb is pleomorphic, such
stains can be difficult to interpret, and mild lymphocytic infiltration is precisely what one would expect in Bb skin lesions,
such as erythema migrans (EM) rash sites.79 Studies that do
mention the search for and identification of pathogens other
than Borrelia spp. are sadly lacking. The methodology in the
study by Altunay et al mentioned “laboratory investigations
including routine biochemical analyses of the blood and
urine, cutaneous biopsy, the microscobic [sic] analysis of
so-called parasites or materials emerging from the skin”.66
DeBonis and Pierre described their microbiological methodology as “Evaluation by a primary care physician revealed
no signs of infection”.61
Many studies included cases that did not meet the
DSM-V criterion for delusional disorder. Some studies
featured patients who were clearly delusional and had or
likely had serious underlying psychiatric illnesses, such as
schizophrenia.62,66 Some described conditions that clearly
indicated the patient had disease affecting the central nervous
system: Roncati et al indicated the patient in their case study
had “myoclonies” [sic], which presumably means seizures or
muscle twitches; one patient in the study by Fellner et al had
senile dementia; and the patient in the report of Freudenreich
et al had HIV infection.58,67,72 Some studies included cases
where so-called delusional disorders could have had cultural
influences.55,66 Some studies mentioned underlying medical
conditions that may have caused psychiatric disturbance.
In the study by Altunay et al, five patients had vitamin B12
deficiency and one had thyroid disease in addition to vitamin
B12 deficiency.66 In the study by Reid and Lio, one patient
had diabetes mellitus.60 Patients in other studies were using
psychoactive drugs.56,61,62,67,70 The patient in the case study by
Roncati et al had hepatitis C virus infection.72
A few case studies claimed that treatment with antipsychotic medication was curative.55,59,60,66 In contrast, many
more case studies indicated that treatment with antipsychotic
drugs reduced symptoms but was not curative55,58,61,66,70,71 or
that antipsychotics were ineffective.62,63,65 In fact, Robles et al
suggested that treatment with antibiotics was more successful than treatment with antipsychotics, although they too
concluded that MD was delusional rather than infectious.
They reported that treatment of two patients with doxycycline
and no antipsychotics resulted in complete resolution of the
condition, while one subject treated with antipsychotics and
no antibiotics did not have disease resolution.65 Some studies
indicated that antipsychotic drugs were prescribed, but failed
to report if treatment was effective,54,55,64,75 and Roncati et
al failed to report what (if any) treatment was prescribed.72
Some of the studies reporting cure or benefit with antipsychotic drugs used other treatment methods in addition to the
antipsychotic medication, and without controls one cannot
be sure which variable was responsible for the patient’s
improvement.55,62,66,67,73
There were only five reports of studies involving larger
cohorts of patients.33,68,69,74,76 Four of these were retrospective
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
79
Morgellons disease
studies,68,69,74,76 and the remaining study selected its cohort by
conducting a retrospective search through medical records.33
Retrospective studies are limited, because data may be
incomplete and cases may lack laboratory analysis or proper
documentation. The retrospective study by Mohandas et al74
included 35 patients and made no mention of the presence
of fibers. The study reported female predominance, with a
mean age of 54.6 years. Psychiatric comorbidities (anxiety
and depression) were noted in 68.5%, and management of
patients included treatment with psychotropic medications
combined with topical and oral antibiotics. Improvement was
reported in less than half of the cohort (40%), and all four
patients who received low-dose oral antibiotic therapy noted
improvement.74 The study by Hylwa et al from the Mayo
Clinic was a retrospective study of psychiatric comorbidity
in a cohort of 54 patients diagnosed with DI. Comorbidities
were found in 74% of these patients, and there was no mention
of fiber or pathogen detection in the retrospective report.69
Foster et al also conducted a retrospective study of DI
at the Mayo Clinic.68 Medical records of 147 patients were
reviewed to determine demographic information, historical
and physical findings, and treatment. In this cohort, 81%
had a history of one or more psychiatric illnesses (the most
common diagnosis being depression), 11% had a history of
drug use (methamphetamines, cocaine, heroin, marijuana,
and other street drugs) that may have contributed to their
symptoms, and only 20% of subjects reported having fibers
in their skin, and thus the cohort was composed predominantly of non-MD subjects. The study lacked fiber analysis,
and there was no mention that any skin-associated fibers
had been visualized by the investigators, so it is possible
that there were no subjects in the study meeting the key
diagnostic MD criterion. Methodology for detecting any
pathogens was lacking, and there was no mention of detection of Borrelia or other tick-borne pathogens at all. Nevertheless, the authors stated that they did not find evidence
of infestation in patient-provided specimens, biopsies, or
tests for ova and parasites.68
The CDC–Kaiser Permanente Northern California–
Armed Forces Institute of Pathology collaborative study
(CDC study) selected their cohort via a retrospective search
through medical records.33 This study had significant flaws.
The case definition did not require the presence of fibers
embedded in or projecting from skin; therefore, selection
was on the basis of self-reported cases, and resulted in a
heterogeneous group of subjects. Eligibility to participate
in the study was limited to those enrolled in a Kaiser Permanente plan. The number of participants diminished as the
study progressed: whereas 467 subjects were identified by a
search of Kaiser Permanente electronic records, cultures for
pathogens were conducted on only 28 subjects, and fibers
were collected from only 12 subjects.33 Fiber analysis was
performed and cotton-textile fibers identified, but the authors
admitted they did not find fibers that were embedded or
projecting from skin, and they admitted that they may have
introduced cotton fibers at the time of sampling. Two of the
subjects identified in the electronic search died, and the cause
of death was not disclosed.
Objective findings of illness that could have accounted for
the symptoms were ignored: cognitive impairment, somatic
complaints, neuropsychiatric symptoms, multiorgan symptoms, and the presence of inflammatory markers.33,80 Cases
with these findings do not meet the DSM-V criteria for
delusional disorder. Most importantly, although the authors
acknowledged that the literature had suggested an association between MD and LD, they did not perform any specific
detection methods, such as Borrelia culture or immunohistochemical staining, for any Borrelia spp., and the search for
LD was limited to insensitive serologic testing. In conclusion,
they used a flawed case definition, selected the wrong cohort,
selected the wrong specimens, performed the wrong tests,
and came to incorrect conclusions with respect to MD and
the association with LD.33,80
Evidence of an infectious etiology
Early history
In modern times, spirochetal infection was implicated as
an etiologic factor for MD as early as 2006, when William
Harvey, a former medical director of a laboratory contracted
to work for NASA, explained to a medical reporter, Chico
Harlan with the Pittsburgh Post-Gazette, that he had been
studying a group of 70 MD patients, all of whom were
infected with Bb, the causative agent of LD.32 Savely et al
in 2006 reported that the principal author, nurse practitioner
Virginia Savely, had seen 80 patients in her practice who
fit the criteria for MD, and all but one of these patients had
tested positive for LD.2
A subsequent study by Harvey et al attempted to delineate MD characteristics in a cohort of 25 self-diagnosed
MD patients.81 Although these patients apparently met the
case definition for DP, the authors felt the cause and effect
of the symptoms were reversed from those of DP, and
they suggested that an infectious process was responsible
for the development of symptoms. They reported that the
male:female ratio was approximately equal, that 23 of 25
subjects had prior psychiatric diagnoses, around 50% had
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
80
Middelveen et al
sensations of movement, 70% had excoriations or lesions, and
that fibers were present in about a third of patients.81 Central
nervous system symptoms, cardiac symptoms, endocrine
dysfunction (hyperparathyroidism, adrenocortical hypofunction, Hashimoto’s thyroiditis, hypercalcemia, elevated fasting
insulin levels, and parathyroid adenomas), a high rate of
autoimmune disease, and low core body temperature were
commonly encountered in their cohort. Laboratory evidence
of abnormalities that were commonly encountered included
anemia, leukopenia, high monocyte count, low natural-killer
cells, elevated serum calcium, elevated globulin levels, and
elevated inflammatory markers (CRP, TNFα, IFNγ). Skin
abnormalities included excoriations, angiomas, and filament/
granule production. The need for a credible MD case definition was emphasized.81
Savely and Stricker analyzed clinical findings in a cohort
of 122 subjects with documented presence of unusual filaments projecting from or embedded in skin.4
The key objective of this study was to develop a credible case definition for
MD, and because cutaneous fibers were the unique objective
finding, the presence of such fibers was determined to be an
obligatory part of the case definition. The link between MD
and LD was explored, and the study reported that 96.8%
of subjects had either positive LD tests by Western blot or
clinical diagnoses of LD; many had positive tests for coinfecting tick-borne illnesses, and the demographics of the
LD patients and MD patients in their practices proved to be
similar. Other important findings in the cohort group were
female predominance and hypothyroidism.4
Middelveen and Stricker provided evidence of spirochetal involvement in the evolution of MD.6
MD was compared to bovine digital dermatitis (BDD), a disease in cattle
caused by various species of treponemes. Several similarities between MD and BDD were noted, including unusual
filament formation, female predominance, rapid spread,
exposure to unsanitary conditions or humid environments,
and positive response to antibiotics. The fact that spirochetes
caused unusual filament formation in cattle suggested that
a similar mechanism might occur in MD patients. The fact
that spirochetes were visible in BDD histological sections
suggested that spirochetes might be present in MD tissue
as well.6
Fiber analysis
Histological studies have shown that filaments in MD tissue
are not textile fibers, but are biofilaments produced by human
epithelial cells and stemming from deeper epidermal layers,
upper dermal layers, and the root sheath of hair follicles
(Figure 1).7,82,83 MD cutaneous filaments are predominantly
composed of keratin and collagen, as determined by histological studies, and appear to be produced by activated keratinocytes and fibroblasts.82,83 The base of filament attachment
to epithelial cells demonstrates nucleation that is continuous
with that of surrounding epithelial cells, indicating that the
filaments are of human cellular origin (Figure 2).83 Histochemical staining of skin sections containing embedded
filaments with Congo red resulted in apple-green birefringence suggestive of an amyloid component, although this
remains to be confirmed using more specific methodologies.7
Calcofluor-white staining of skin sections with embedded
filaments was negative, and thus MD filaments do not have
any cellulose content from plant fibers, such as cotton, or
chitin from fungal cells or insect exoskeletons.7
Figure 1 Embedded cutaneous blue and white filaments.
Notes: Note elaborate arrangement with branching and tapered ends; magnification
50×.
Figure 2 Longitudinal sections of filaments originating in the basal layer of the
epidermis adjacent to the dermis; magnification 400×.
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
81
Morgellons disease
Several independent studies have shown that blue MD
fibers were human hairs or hairlike extrusions and that blue
coloration resulted from melanin pigmentation (Figure 3).
Blue textile fibers are colored by dyes, not by blue melanin
pigmentation; therefore, it is not possible that blue MD fibers
are textile in origin. MD filaments are hairlike extrusions, and
some MD fibers are very fine human hairs.7,82,83 The coloration
of blue fibers was shown to result from melanin pigmentation,
which was demonstrated by positive histochemical staining
with Fontana Masson. A confirmatory study performed at a
laboratory specializing in biofibers and coloration established
that embedded blue fibers in MD dermatological specimens
were human hairs.
SEM of blue MD fibers shows cuticular scaling consistent
with human hairs, and transmission electron microscopy
shows darkly stained, disorganized melanosomes consistent
with human hairs.7,83 Microspectrophotometry reflectance of
blue fibers is consistent with that of pigmented tissues, and
Raman spectroscopy results in relevant peaks corresponding
to carbamate compounds and melanin aromatic rings (MD
Shawkey, University of Akron, personal communication,
2013).7
An investigation concluded that fibers were not selfimplanted, due to the fact that they were deeply embedded in
skin in a manner that a patient would not be able to achieve
(MD Shawkey, University of Akron, personal communication, 2013).
Other MD findings
If MD specimens are examined, they demonstrate evidence
of abnormal keratin and collagen expression. In addition to
the formation of abnormal cutaneous fibers, many patients
report changes to hair and fingernails.82 Deformed follicular
bulbs, pili multigemini (formation of multiple hair shafts
within individual follicles), filamentous projections from the
follicular sheath surrounding hair bulbs, and the formation of
thickened keratin projections are common findings.82,83 The
authors of this paper have had the opportunity to examine
many MD lesions and MD dermatological specimens (Fesler,
Middelveen, and Stricker, unpublished data, 2017). We have
noted that MD lesions can begin as folliculitis that evolves
into ulcerative filamentous lesions, with further evidence
of keratin and collagen abnormalities, such as formation
of keratin projections, formation of hardened comedo-like
masses, and deformities of hairs and hair follicles, as mentioned previously.
Keratin projections are thickened follicular casts. When
sectioned and stained with Gömöri trichrome, these follicular casts are abnormal in that although the outer surface
is composed of keratin-rich tissue, the interior can contain
collagen-rich tissue. Comedo-like masses can emerge from
pores spontaneously or when scratched, and are sometimes
described by patients as being sand-like. Patients may misinterpret these objects as being seeds, eggs, cocoons, parasites,
or even arthropods. These comedo-like masses can contain
embedded keratin or collagen filaments and/or projecting
filaments. When they form inside a pore or follicle, they may
form a tight wad of fibers (Figure 4A). Hair and follicular
bulb deformities include pili multigemini (mentioned previously) (Figure 4B), hairs or fibers growing downward deep
into the dermis rather than in the opposite direction through
the pore opening, and follicular sheaths with filamentous projections. These projections can completely cover the follicular
sheath, and may be interpreted as caterpillars by patients.
Contaminating extraneous artifacts can complicate
identification of legitimate dermatological findings in MD.
We have found pollen, noninfesting arthropods, feathers,
and mites in patient-supplied specimens. Some patients
have described the production of “fuzzballs”. We performed
histological sectioning and staining with Gömöri trichrome
on such artifacts and determined the “fuzzballs” in patientsupplied samples that we studied were largely composed of
textile fibers, but did have some keratin fibers or keratinized
tissue present as well. We speculate that such artifacts may
include keratin-containing material related to MD, but that
textile fibers can attach to sticky exudate or can tangle into
filamentous lesions. Other patients have described hexagonal
crystals and “glitter” in MD skin. Spectroscopic analysis of
the hexagonal crystals proved that they were contaminating
man-made hexagonal objects (of the type used in cosmetics
Figure 3 Filaments remaining embedded in deeper layers of skin after removal of a
callus; magnification 100×.
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
82
Middelveen et al
and greeting cards) with either cellulose or plastic centers
and a metallic coating (V Loyd, Mount Allison University,
personal communication, 2017). The “glitter” that we studied
contained salts that were likely human bioproducts, and may
have a role in MD (unpublished data). As extraneous artifacts
can contaminate sticky lesions, it is important to collect
only fibers deeply embedded in skin or clearly projecting
in a hair-like manner for studies intended to determine fiber
composition.
As mentioned earlier in the report, cattle with BDD form
lesions that produce unusual keratin projections. Although
visually different from the small slender filaments in MD
lesions, we have also observed slender fibers in BDD
specimens that strongly resemble the fibers in human MD
(Figure 5) (unpublished observation).
Detection of pathogens
Early on in MD history, a link between MD and LD was
reported.1–7 Savely and Stricker reported that 96.8% of their
cohort of 122 MD patients had either positive LD serology or
an LD diagnosis.4
A more recent study reported that 6% of LD
patients in a cohort of Australian LD subjects had MD,84 and
Borrelia spirochetes have repeatedly been detected in skin
and bodily fluid specimens from MD subjects. Preliminary
studies reported that Bb sensu stricto (Bbss) spirochetes were
detected in dermatological tissue removed from MD lesions
of four North American patients,83,85 and an ensuing study
reported the detection of Borrelia garinii in MD samples
from an Australian patient.86
A larger study was needed to verify the association
between Borrelia infection and MD. Consequently, a study
of 25 North American MD patients confirmed the presence of Borrelia spirochetes in MD-tissue and body-fluid
specimens, both directly in dermatological specimens and in
cultures obtained from MD patients using microscopic, histopathological, and molecular detection methods.87 This study
provided evidence for the presence of Borrelia DNA in MD
specimens by PCR followed by DNA sequencing performed
by two independent laboratories. PCR technology amplified Borrelia DNA in 13 MD whole-callus specimens (nine
sequenced), four cultures inoculated with dermatological
tissue (one sequenced), eight blood cultures (two sequenced),
two vaginal secretion cultures (both sequenced), and one
intestinal specimen. The Borrelia spirochetes detected in
these studies were identified primarily as Bbss, but B. garinii
and B. miyamotoi were also reported.87
The fact that motile spirochetes identified as Borrelia spp.
were detected in Barbour–Stoenner–Kelly H medium inoculated with MD dermatological tissue proves that spirochetes
present in MD tissue are viable.85,87 Identification of Borrelia
spirochetes in cultures is complicated by fastidious growth
requirements and pleomorphism,88,89 but PCR amplification
of cultured spirochetes in these studies provided confirmatory
molecular identification of live Borrelia spirochetes in specimens from MD subjects.87 Four laboratories independently
confirmed the presence of Borrelia DNA in MD specimens
using PCR technology and confirmatory DNA sequencing.7
Recently, independent researchers from Canada using PCR
technology and confirmatory DNA sequencing have detected
Borrelia DNA in cultures inoculated with specimens from
MD patients (J Lewis, V Lloyd, Mount Allison University,
personal communication, 2017). Therefore, five laboratories
using PCR technology have now provided confirmation of
Borrelia DNA in MD specimens, including four species of
Borrelia: Bbss, B. garinii, B. hermsii, and B. miyamotoi7,90,91(J
Lewis, V Lloyd, Mount Allison University, personal communication, 2017). The detection of Borrelia spirochetes is
reproducible, providing that correct methods of detection are
employed (Figure 6).
Borrelia spirochetes can invade and replicate inside
fibroblasts and keratinocytes,92–94 and have been isolated in
vitro from monolayers of keratinocytes and fibroblasts despite
antibiotic treatment.92,93 Sequestration in these cells may be a
contributing factor in the development of refractory infection
Figure 4 (A) A filamentous follicular cast. White filaments originating on the outer follicular sheath are growing in a coiled manner. Magnification 50×. (B) Pili multigemini,
a common finding in Morgellons disease patients, with multiple hairs forming from a single bulb. Magnification 50×.
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
83
Morgellons disease
in MD patients. In support of that hypothesis, Borrelia
spirochetes were detected in MD skin specimens removed
from patients treated with aggressive antibiotic therapy.87
Hypothetically, intracellular infection of keratinocytes and
fibroblasts would be able to alter keratin and collagen gene
expression, respectively, resulting in unusual filament formation.7
Some patients report that gel was secreted from their
skin. We sectioned these dried-gel secretions, and immunostaining targeted to Borrelia was positive and demonstrated a
copious quantity of well-formed spirochetes embedded in a
clear matrix. This finding is consistent with biofilm formation
(unpublished data). The spirochetal load in MD specimens
is high and suggests biofilm formation in vivo.85,87 The formation of biofilms may also contribute to the severity of the
dermopathy and antibiotic resistance.
The key etiologic factor contributing to the evolution
of MD lesions seems to be infection with Borrelia spp.,
the pathogen most consistently detected in MD patients.
Figure 5 (A) Thickened keratinized follicular casts in a Morgellons disease specimen that grew inward into the dermis. Note the clear inward-growing hair. Magnification
100×. (B) Specimen from a bovine digital dermatitis lesion with similarities to human Morgellons specimens. Note thickened keratin projections and the threadlike blue
filament (lower part of specimen). Magnification 50×.
A
B
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
84
Middelveen et al
However, we speculate that the etiology of MD is multifactorial. Factors such as genetic predisposition, endocrine influences, immune status, and the presence of other infections,
particularly tick-borne coinfections, appear to play a role
in the development of this phenomenon.1–7 Pathogens other
than Borrelia spp. have been detected in MD tissue samples,
including Helicobacter pylori, Treponema denticola, and
Bartonella henselae.
87,90,91,95 Preliminary genetic studies have
demonstrated nine genes with significant sequence variation
in MD patients (E Sapi, University of New Haven, unpublished observation, 2017). Examination of genetic factors
that contribute to MD is currently in progress.
Recently, MD-like filamentous dermatitis was described
in domestic dogs, and Bbss was detected by PCR amplification confirmed by DNA sequencing, thus providing evidence
that MD-like filamentous dermatitis may be associated with
LD in these dogs.96 Interestingly, many of these dogs were
bulldogs or other breeds with color-dilution genes, thus suggesting that genetics may predispose certain breeds of dogs
infected with Borrelia to develop this skin condition. Many of
the owners of these pets had no prior knowledge of MD, and
thus these were not cases of delusion by proxy.96 The fact that
a condition analogous to MD in humans can occur in dogs
and a similar animal model of spirochetal infection associated
with filament formation – BDD – occurs in cattle provides
supportive evidence that MD is an infectious process.
Antibiotic treatment of MD
Although there is anecdotal evidence that MD responds to
antibiotics,1–7 controlled studies of MD treatment with antibiotics have not been conducted. Optimal treatment for MD
remains undetermined. Two of the authors report success in
treating LD/MD patients with antibiotics. In general, early
treatment contributes to a better patient outcome. Treatment
aimed at the underlying tick-borne disease is essential to
resolve MD dermopathy, and treatment may require both
prolonged combination-antibiotic therapy and the identification and treatment of any coinfecting tick-borne diseases
or other exacerbating factors. Some patients benefit from
antiparasitic therapy, although there appears to be no direct
evidence of parasite infection in MD.
Clinical classification of MD
A clinical classification scheme has been proposed for MD:
1. early localized: lesions/fibers present for less than 3
months and localized to one area of the body (head, trunk,
extremities)
2. early disseminated: lesions/fibers present for less than
3 months and involving more than one area of the body
(head, trunk, extremities)
3. late localized: lesions/fibers present for more than 6
months and localized to one area of the body (head, trunk,
extremities)
4. late disseminated: lesions/fibers present for more than
6 months and involving more than one area of the body
(head, trunk, extremities).
This classification scheme centers on the duration and location of MD lesions with the intent to validate and standardize
the diagnosis of MD.7,87
Discussion
The diagnosis of Delusional disorder 297.1 (F22), somatic
type, as described in the DSM-V, requires clinical judgment, as
the delusional belief should not be better explained by another
mental disorder, be caused by the effects of a substance or
medication, nor caused by other medical conditions, such as
infection. Many of the case studies cited in this paper mentioning MD concern patients who have medical conditions such
as diabetes, vitamin B12 deficiency, substance-abuse problems,
and infections that could be implicated in the development of
their symptoms, and diagnosing such patients with delusional
disorder is contrary to DSM-V principles. The diagnosis of
a delusional disorder is best made by a professional with
mental health training, such as a psychiatrist. Single, isolated
delusions are quite rare, and truly delusional patients have
evidence of other delusions, not just DOP.
There is significant overlap in the array of symptoms
that may accompany LD, MD, and mental illness, thus
complicating the diagnosis. In theory, patients who do not
Figure 6 Single spirochete from a Morgellons disease skin specimen immunostained
for detection of Borrelia. Magnification 1,000×.
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
85
Morgellons disease
have MD but who are delusional could think they have MD
if they have had exposure to the topic through the Internet
or other means.7,87 To complicate the diagnosis further,
MD patients may exhibit neuropsychiatric symptoms, and
many have psychiatric diagnoses, such as bipolar disorder,
attention-deficit disorder, obsessive compulsive disorder,
and schizophrenia.1,7,81 Therefore, many MD patients may
have psychiatric comorbidities, and in some cases, patients
have been misdiagnosed with a psychiatric illness that they
do not have.7
Some MD patients may have false beliefs that
are not delusional in origin. Lack of scientific knowledge can
cause patients to misinterpret symptoms, such as the presence of filaments and sensations of formication as worms,
arthropods, or other infestations. In addition, MD lesions
are sticky and arthropods or artifacts can adhere to exudate,
and patients may incorrectly believe these external factors
are associated with the dermopathy.7,87
A patient who (because of symptom misinterpretation or
lack of scientific knowledge) believes he or she has a parasitic
infestation should not be diagnosed with delusional mental
illness. It is logical for a patient to speculate that a complex
of symptoms, including abnormal skin fibers coupled with
formication, could be caused by a parasite. Furthermore,
patients with MD are not always aware that they have
filaments, because magnification is needed to visualize the
filaments and many are diagnosed with other conditions,
such as lichen sclerosus or prurigo nodularis, which lack
filament formation.7,87 In addition, systemic LD is commonly
associated with dermatological conditions and neurological
symptoms, such as paresthesias.7,87,97,98 As such, multiorgan
MD symptoms overlap with LD because MD is associated
with LD, and this dermatological and neurological symptom
overlap may partly explain the odd movement or stinging
sensations that MD patients experience.
The DSM-V does not mention DOP, DI, or DP. In the
case of Delusional disorder 297.1 (F22), delusions are not
the beliefs themselves, but the way they are interpreted by
patients. Delusions are profound, intensely held beliefs that
seem barely swayed by evidence to the contrary, even to the
point of believing in the bizarre.34–36 In contrast, the presence of human biofibers embedded in skin of MD patients
is factual: the patients are not hallucinating imaginary fibers,
and they are not implanting textile fibers or hallucinating an
imaginary infestation. MD is not a case of fixed belief despite
lack of medical evidence, because if fibers are present and
they are visible under magnification, then there is medical
evidence. MD fibers projecting from and embedded in skin
may have elaborate configurations with branching, and the
filaments may have tapered ends. Even skilled microsurgeons
could not implant the fibers in that configuration.
The evidence is present in patients who have MD, but in
order to be recognized, a physician must be willing to look.
If patients meet the case definition for MD with visible skin
fibers and do not believe they are infested, these patients do
not meet the criteria for a diagnosis of Delusional disorder
297.1, somatic type, as described in the DSM-V. Creeping
and crawling sensations unaccompanied by delusions of
infestation are not enough to give a patient a diagnosis of
delusional disorder. These sensations are consistent with the
well-recognized symptom of formication that occurs with
peripheral neuropathy and is associated with many medical
conditions, such as diabetes, chronic infections, menopause,
skin cancer, and multiple sclerosis, and exposure to various
chemical substances, such as toxins, certain medications,
alcohol, or recreational drugs. It is contrary to proper psychiatric diagnosis to label a patient as having a delusional
mental illness based solely on a complaint of formication.
LD and associated tick-borne diseases may be accompanied by mental illness.99–101 Chronic progressive neurodegenerative diseases can be caused by infection and
resulting prolonged inflammation.101,102 The spectrum of
mental illnesses associated with LD varies in severity,
and includes anxiety, depression, paranoia, schizophrenia,
bipolar disorder, sensory hallucinations, and homicidal
tendencies.101,103 Some of these neuropsychiatric conditions
involve a delusional component. Delusions resulting from
infectious processes do not meet the DSM-V criteria for
delusional disorder. Furthermore, the presence of psychiatric
comorbidities is not proof that a patient is delusional. Some
patients have a component of posttraumatic stress disorder
and are hypervigilant and overreactive to physical symptoms,
rather than being delusional. If a health care provider cannot tell the difference between a hypervigilant patient and a
delusional patient, the provider is not qualified to diagnose
delusional disorder.
The presentation of specimens or pictorial evidence to a
doctor is not an indication of delusional disorder or mental
illness. This action was never included as an indication of
delusional disorder in the DSM-V. Likewise, the fact that a
patient with beliefs of infestation accompanied by movement sensation has psychiatric comorbidities69,74 is not proof
of delusional disorder and is contrary to the recommended
practices of the DSM-V.80 In most of the case studies that
equate MD with DOP, DP, or DI, patient-supplied evidence
was dismissed, evidence of disease (physical and laboratory) was dismissed, fibers were identified as being textile
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
86
Middelveen et al
in origin based solely on visual examination, physicians
were unwilling to examine skin at sufficient magnification
to see microscopic fibers, and all too quickly patients were
diagnosed as being delusional in a manner that is contrary
to the DSM-V approach to psychiatric diagnosis.
The bar set for burden of proof is higher for those proposing an infectious etiology than for those proposing that MD
is delusional. Many of the papers reviewed in this analysis
relied solely on visual identification for “textile fibers”. Others did not select the correct patients to study (ie, patients
with documented embedded or projecting cutaneous filaments) or did not collect the correct specimens, collecting
instead superficial artifacts, including some that might have
been introduced at the time of sampling. The analysis of
MD fibers requires that the patient meets the case definition
with the key diagnostic criterion of having colored, white,
or black filaments protruding from or embedded in skin, and
the correct specimens must be collected.
Fibers that are embedded in deeper layers of skin or that
are firmly attached, originating underneath the stratum corneum and projecting either outward to the surface or inward
into the dermis, are the only specimens that are suitable for
fiber analysis. Most often, MD fibers are present as inclusions in callus material that resembles scabs. These can be
removed for analysis by a health care practitioner, or in rare
cases patient-supplied specimens of calluses can contain
fibers suitable for analysis, provided that calluses composed
of skin and fibers are embedded throughout the specimen.
Histological sectioning and staining to detect keratin and
collagen can visually and chemically determine the keratin
and collagen nature of these fibers.7,83
When diagnosing mental illness, it is imperative first to
determine if there is an underlying cause of the psychiatric
symptom, such as an infection. None of the case studies
reviewed in this paper or the research studies involving larger
cohorts of MD patients looked adequately for infections, in
particular LD. Science has to be reproducible, and there has
to be enough detail provided in the methodology description
for the study to be replicated. This was not the case for detecting LD in many of the case studies. Borrelia spirochetes are
readily detectable in MD tissue, but sensitive and specific
methods are required.7,87 Although sensitive and specific
direct-detection methods, such as antigen detection, culture
of Borrelia spirochetes, and PCR detection of Borrelia
DNA, exist, these methods are not standardized, and vary in
sensitivity and specificity.104,105 They are not recommended
by the CDC, which only endorses two-tier serological LD
testing.7,87,106 Unfortunately, two-tier serological testing for
LD, although specific for Bbss, lacks sensitivity and is little
better than a coin toss in detecting LD.107,108
False negatives can occur when using two-tier testing for
a number of reasons, including the fact that some patients
with known LD are seronegative.107,109 In addition, there is
significant genetic diversity in Borrelia spp. capable of causing LD and LD-like illnesses, but commercial two-tier testing
is based on the antigens of one laboratory strain, and testing
may not detect other Borrelia species.110–112 The fact that the
CDC does not consider any direct detection method, not even
culture, as being diagnostic for LD as proof of infection is
unjustifiable. It should be noted that culture is considered
to be the gold standard for detection of organisms by the
American Society for Microbiology.113 The reluctance of the
CDC to accept more sensitive testing methods for LD makes
the evidence showing the association between LD and MD
controversial.7,87 Those who maintain that MD is a form of
DOP or DI and rely on the two-tier test for Borrelia detection
claim that studies supporting an infectious etiology and an
association with LD are flawed,72,75 yet these critics have not
used adequate methodologies, and by failing to do so have
not proved that methods for detecting Borrelia spp. used in
more sophisticated studies are unreproducible or false.
Patients diagnosed with DOP in case studies are frequently prescribed antipsychotic medication with potentially
serious side effects.30 These patients are often talked into taking antipsychotic medication by health care providers using
deceptive unethical dialogue, which can compromise patient
autonomy.41,43,50,54,60,114 Many published articles mentioning
MD make claims that antipsychotic drugs are effective treatment for MD, but careful review of the literature suggests
otherwise. Two systematic reviews concerning the use of
these medications to treat DI, DOP, or DP cases conclude
that treatment efficacy was unproven.115,116 In many of the
case studies reviewed in this paper, authors claimed that
antipsychotics were used to treat MD cases, yet the patients
received treatment in addition to antipsychotic drugs, including antibiotics, wound dressings, antiseptics, and antipruritic
drugs.59,65,66 Therefore, it is not certain which treatments were
providing benefit, as the studies were uncontrolled. In fact,
one study reported complete remission using antibiotics and
not with antipsychotics.65
Antipsychotic medications can have off-label effects,
such as reduced growth of parasites and anti-pruritic properties.117 Evidence showing an association between antipsychotic treatment in DI, DOP, or DP patients and resolution
of symptoms or benefit is very limited and is more limited in
MD cases. There has been only one randomized, double-blind,
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
87
Morgellons disease
placebo-controlled study that evaluated the effectiveness of
the antipsychotic drug pimozide.118 This study of a small
cohort of eleven DI patients reported that pimozide was better
than placebo at controlling formication, but was not better at
controlling delusions of vermin infestation or excoriation.118
One study of 14 DI/DOP/DP patients reported that although
seven patients remained in remission 19–48 months after
pimozide treatment, four patients had no response to this
antipsychotic medication.119 The variation seen in reported
effectiveness in this study and the various studies reviewed
here may have arisen from the fact that patients diagnosed
with DI are a heterogeneous group of individuals, some of
whom are truly delusional and some of whom are not.
Recent studies using advanced brain magnetic resonance
imaging (MRI) technology have found that patients diagnosed with DI have significant gray-matter changes that differ
from findings in both patients with nonsomatic delusions
and healthy controls.120–122 These MRI abnormalities involve
altered cortical thickness and surface area in various parts of
the brain, indicating that selective delusional symptoms in
patients may be based on specific somatic brain alterations. It
is tempting to speculate that these brain alterations are related
to spirochetal infection in MD, either via direct brain invasion or an inflammatory response in genetically susceptible
individuals.123,124 The intriguing link between spirochetal
infection and brain pathology detected by advanced imaging
methods merits further study.
Conclusion
The history of MD has taught us that scientific evidence
must be carefully considered before a disease is written off
as a purely psychiatric disorder. Delusional disorder is a
diagnosis of exclusion that requires clinical judgment, and
all underlying causes for delusional symptoms need to be
ruled out before jumping to erroneous conclusions. Medical
practitioners continue to consider MD a delusional disorder,
although studies have shown that MD is strongly associated
with spirochetal infection. According to the best-available
scientific evidence, MD should be considered a dermopathy
associated with tick-borne disease. Further study of the
genetics, pathogenesis, and treatment of MD is warranted.
Acknowledgments
The authors thank Jesus Walker-Salas, Diana Canchola and
Jeannie Ramos for technical assistance. This work was supported in part by a grant from the Lindorf Family Foundation,
Newark, OH.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Savely G, Leitao MM. Skin lesions and crawling sensations: disease
or delusion? Adv Nurse Pract. 2005;13(5):16–17.
2. Savely VR, Leitao MM, Stricker RB. The mystery of Morgellons
disease: infection or delusion? Am J Clin Dermatol. 2006;7(1):1–5.
3. Savely VR, Stricker RB. Morgellons disease: the mystery unfolds.
Expert Rev Dermatol. 2007;2(5):585–591.
4. Savely VR, Stricker RB. Morgellons disease: analysis of a population
with clinically confirmed microscopic subcutaneous fibers of unknown
etiology. Clin Cosmet Investig Dermatol. 2010;3:67–78.
5. Leland DK. Touched by Lyme: threading your way through Morgellons
disease. 2017. Available from: https://www.lymedisease.org/touchedlyme-threading-path-morgellons-disease. Accessed September 9,
2017.
6. Middelveen MJ, Stricker RB. Filament formation associated with
spirochetal infection: a comparative approach to Morgellons disease.
Clin Cosmet Investig Dermatol. 2011;4:167–177.
7. Middelveen MJ, Stricker RB. Morgellons disease: a filamentous borrelial dermatitis. Int J Gen Med. 2016;9:349–354.
8. Kellett CE. Sir Thomas Browne and the disease called Morgellons.
Ann Med Hist. 1935;7:467–479.
9. Thibierge G. Les acaraphobes. J Prat Rev Gen Clin Ther. 1894;8:
373–376.
10. Lee WR. The matchbox sign. Lancet. 1983;2(8347):457–458.
11. Wilson JW, Miller HE. Delusion of parasitosis (acarophobia). Arch
Derm Syphilol. 1946;54:39–56.
12. Raecke J. Uber hypochondrie. Allg Z Psychiatr. 1902;59:319–410.
13. Grøn K. Les dermatophobies. Poster presented at: 6th meeting of the
Nordic Dermatological Association; August 26–28, 1924; Helsinki,
Finland.
14. MacNamara ED. Cutaneous and visual hallucinations. Lancet.
1928;214:807–808.
15. Eller JJ. Neurogenic and psychogenic disorders of the skin. Med J Rec.
1929;129:481–485, 554–559, 616–620, 675–679.
16. Schwarz H. Circumscripte hypochondrien. Monatsschr Psychiatr
Neurol. 1929;72:150–164.
17. Mallet R, Male P. Délire cénesthesique. Ann Med Psychol (Paris).
1930;88:198–201.
18. Vié J. L’idée délirante d’anthropathie interne. Poster presented at:
39th Congress of Alienist Physicians and Neurologists of France; July
22–28, 1935; Brussels, Belgium
19. Klauder JV. Psychogenic aspects of skin diseases. J Nerv Ment Dis.
1936;84(3):249–273.
20. Ekbom KA. Praeseniler dermat-zooenwahn. Acta Psychiatr Scand.
1938;13:227–259.
21. Kohl PK, Winzer I. The 100 years since discovery of Spirochaeta
pallida. Hautarzt. 2005;56(2):112–115.
22. Bigger JW. The reliability of the Wassermann test as performed by
different pathologists. J Hyg (Lond). 1921;20(4):383–389.
23. Gilbert R. Standardization of the Wasserman test. Am J Public Health
Nations Health. 1930;20(1):47–48.
24. Kean WF, Toccio S, Kean M, Rainsford KD. The musculoskeletal
abnormalities of the Similaun iceman (Ötzi): clues to chronic pain
and possible treatments. Inflammopharmacology. 2013;21(1):11–20.
25. Marshall WF, Telford SR, Rys PN, et al. Detection of Borrelia burgdorferi DNA in museum specimens of Peromyscus leucopus. J Infect
Dis. 1994;170(4):1027–32.
26. Poinar G. Spirochete-like cells in a Dominican amber Amblyomma tick
(Arachnida: Ixodidae). Hist Biol. 2015;27(5):565–570.
27. Emslie-Smith AH. Myiasis, fillan, and the morgellons. Br Med J.
1946;1:962.
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
88
Middelveen et al
28. Lyell A. Delusions of parasitosis. J Am Acad Dermatol. 1983;8(6):
895–897.
29. Robles DT. Morgellons disease and the ‘tweezer sign’. Clin Exp
Dermatol. 2008;33(6):793–794.
30. Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol
Rev. 2009;22(4):690–732.
31. Freudenmann RW, Kölle M, Schönfeldt-Lecuona C, Dieckmann
S, Harth W, Lepping P. Delusional parasitosis and the matchbox
sign revisited: the international perspective. Acta Derm Venereol.
2010;90(5):517–519.
32. Harlan C. Mom fights for answers on what’s wrong with her son.
2006. Available from: http://www.post-gazette.com/local/2006/07/23/
Mom-fights-for-answers-on-what-s-wrong-with-her-son/stories/200607230221. Accessed August 4, 2017.
33. Pearson ML, Selby JV, Katz KA, et al. Clinical, epidemiologic, histopathologic and molecular features of an unexplained dermopathy.
PLoS One. 2012;7(1):e29908.
34. American Psychiatric Association. Diagnostic and Statistical Manual
of Mental Disorders. 5th ed. St Louis: APA; 2016.
35. Manschreck TC. Delusional disorder: the recognition and management
of paranoia. J Clin Psychiatry. 1996;57 Suppl 3:32–38; discussion 49.
36. Fennig S, Fochtmann LJ, Bromet EJ. Delusional and shared psychotic
disorder. In: Sadock BJ, Sadock VA, editors. Kaplan and Sadock’s
Comprehensive Textbook of Psychiatry. 8th ed. Philadelphia: LWW;
2005:1525–1533.
37. Bourgeois JA, Khan RA, Hilty DM. Delusional disorder. 2015. Available from: http://emedicine.medscape.com/article/292991-overview.
Accessed September 4, 2017.
38. Koblenzer CS. Pimozide at least as safe and perhaps more effective
than olanzapine for treatment of Morgellons disease. Arch Dermatol.
2006;142(10):1364.
39. Koblenzer CS. The challenge of Morgellons disease. J Am Acad Dermatol. 2006;55(5):920–922.
40. Waddell AG, Burke WA. Morgellons disease? J Am Acad Dermatol.
2006;55(5):914–915.
41. Accordino RE, Engler D, Ginsburg IH, Koo J. Morgellons disease?
Dermatol Ther. 2008;21(1):8–12.
42. Molyneux J. AKA ‘Morgellons’. Am J Nurs. 2008;108(5):25–26.
43. Robles DT, Romm S, Combs H, Olson J, Kirby P. Delusional disorders
in dermatology: a brief review. Dermatol Online J. 2008;14(6):2.
44. Lustig A, Mackay S, Strauss J. Morgellons disease as Internet meme.
Psychosomatics. 2009;50(1):90.
45. Wildner M. [Do they understand Morgellons disease?] Gesundheitswesen. 2009;71(12):795–796. German.
46. Fair B. Morgellons: contested illness, diagnostic compromise and
medicalisation. Sociol Health Illn. 2010;32(4):597–612.
47. Halvorson CR. An approach to the evaluation of delusional infestation.
Cutis. 2012;90(4):E1–E4.
48. Schmidt E, Levitt J. Dermatologic infestations. Int J Dermatol.
2012;51(2):131–141.
49. Misery L. Morgellons syndrome: a disease transmitted via the media.
Ann Dermatol Venereol. 2013;140(1):59–62.
50. Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31(1):31–40.
51. Wong JW, Koo JY. Delusions of parasitosis. Indian J Dermatol.
2013;58(1):49–52.
52. Vulink NC. Delusional infestation: state of the art. Acta Derm Venereol.
2016;96(217):58–63.
53. Ferreira BR, Roccia MG, Cardoso JC, et al. History of Morgellons
disease: the same name for different psychodermatologic diseases?
Wien Med Wochenschr. 2017;167(Suppl 1):49–51.
54. Murase JE, Wu JJ, Koo J. Morgellons disease: a rapport-enhancing
term for delusions of parasitosis. J Am Acad Dermatol. 2006;55(5):
913–4.
55. Bhandary SK, Peter R, Bhat S. Delusional parasitosis in ENT. Indian
J Otolaryngol Head Neck Surg. 2008;60(4):387–9.
56. Vila-Rodriguez F, Macewan BG. Delusional parasitosis facilitated
by web-based dissemination. Am J Psychiatry. 2008;165(12):1612.
57. Dovigi AJ. Intraoral Morgellons disease or delusional parasitosis: a
first case report. Am J Dermatopathol. 2010;32(6):603–605.
58. Freudenreich O, Kontos N, Tranulis C, Cather C. Morgellons disease,
or antipsychotic-responsive delusional parasitosis, in an HIV patient:
beliefs in the age of the Internet. Psychosomatics. 2010;51(6):453–457.
59. Harth W, Hermes B, Freudenmann RW. Morgellons in dermatology.
J Dtsch Dermatol Ges. 2010;8(4):234–242.
60. Reid EE, Lio PA. Successful treatment of Morgellons disease with
pimozide therapy. Arch Dermatol. 2010;146(10):1191–1193.
61. DeBonis K, Pierre JM. Psychosis, ivermectin toxicity, and “Morgellons
disease”. Psychosomatics. 2011;52(3):295–296.
62. Dewan P, Miller J, Musters C, Taylor RE, Bewley AP. Delusional
infestation with unusual pathogens: a report of three cases. Clin Exp
Dermatol. 2011;36(7):745–748.
63. Gartner AM, Dolan SL, Stanford MS, Elkins GR. Hypnosis in the
treatment of Morgellons disease: a case study. Int J Clin Exp Hypn.
2011;59(2):242–249.
64. Grosskopf C, Desai B, Stoopler ET. An oral ulceration associated with
Morgellons disease: a case report. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 2011;112(2):e19–e23.
65. Robles DT, Olson JM, Combs H, Romm S, Kirby P. Morgellons disease
and delusions of parasitosis. Am J Clin Dermatol. 2011;12(1):1–6.
66. Altunay IK, Ates B, Mercan S, Demirci GT, Kayaoglu S. Variable
clinical presentations of secondary delusional infestation: an experience of six cases from a psychodermatology clinic. Int J Psychiatry
Med. 2012;44(4):335–350.
67. Fellner MJ. New findings in delusions of parasitosis. Skinmed.
2012;10(2):72–74.
68. Foster AA, Hylwa SA, Bury JE, Davis MD, Pittelkow MR, Bostwick
JM. Delusional infestation: clinical presentation in 147 patients seen
at Mayo Clinic. J Am Acad Dermatol. 2012;67(4):673.e1–10.
69. Hylwa SA, Foster AA, Bury JE, Davis MD, Pittelkow MR, Bostwick
JM. Delusional infestation is typically comorbid with other psychiatric
diagnoses: review of 54 patients receiving psychiatric evaluation at
Mayo Clinic. Psychosomatics. 2012;53(3):258–265.
70. Mortillaro G, Rodgman C, Kinzie E, Ryals S. A case report highlighting the growing trend of Internet-based self-diagnosis of “Morgellon’s
disease”. J La State Med Soc. 2013;165(6):334–336.
71. Ranka N, Godse K, Nadkarni N, Patil S, Agarwal S. Morgellons
disease: a myth or reality? Indian Dermatol Online J. 2016;7(5):
430–432.
72. Roncati L, Gatti AM, Pusiol T, Piscioli F, Barbolini G, Maiorana A.
The first investigative science-based evidence of Morgellons psychogenesis. Ultrastruct Pathol. 2016;40(5):249–253.
73. Sandhu RK, Steele EA. Morgellons disease presenting as an eyelid
lesion. Ophthal Plast Reconstr Surg. 2016;32(4):e85–e87.
74. Mohandas P, Bewley A, Taylor R. Morgellons disease: experiences of
an integrated multidisciplinary dermatology team to achieve positive
outcomes. J Dermatolog Treat. Epub 2017 Jul 14.
75. Ohn J, Park SY, Moon J, Choe YS, Kim KH. Morgellons disease. Ann
Dermatol. 2017;29(2):223–225.
76. Shah R, Taylor RE, Bewley A. Exploring the psychological profile of patients with delusional infestation. Acta Derm Venereol.
2017;97(1):98–101.
77. Cargnello JA, Powell JJ, Thompson RP, Crocker PR, Watt F. Elemental
hair analysis using nuclear microscopy and X-ray energy dispersive
spectroscopy. Analyst. 1995;120:783–787.
78. Kruszelnicki KS. Dental floss 1. 2001. Available from: http://www.abc.
net.au/science/articles/2001/03/30/268342.htm. Accessed September
9, 2017.
79. Böer A, Bresch M, Dayrit J, Falk TM. Erythema migrans: a reassessment of diagnostic criteria for early cutaneous manifestations of
borreliosis with particular emphasis on clonality investigations. Br J
Dermatol. 2007;156(6):1263–1271.
Clinical, Cosmetic and Investigational Dermatology 2018:11 submit your manuscript | www.dovepress.com
Dovepress
Dovepress
89
Morgellons disease
80. Stricker RB, Middelveen MJ. Morgellons disease: more questions than
answers. Psychosomatics. 2012;53(5):504–505.
81. Harvey WT, Bransfield RC, Mercer DE, Wright AJ, Ricchi RM, Leitao
MM. Morgellons disease, illuminating an undefined illness: a case
series. J Med Case Rep. 2009;3:8243.
82. Middelveen MJ, Rasmussen EH, Kahn DG, Stricker RB. Morgellons
disease: a chemical and light microscopic study. J Clin Exp Dermatol
Res. 2012;3:140.
83. Middelveen MJ, Mayne PJ, Kahn DG, Stricker RB. Characterization
and evolution of dermal filaments from patients with Morgellons
disease. Clin Cosmet Investig Dermatol. 2013;6:1–21.
84. Mayne PJ. Clinical determinants of Lyme borreliosis, babesiosis,
bartonellosis, anaplasmosis, and ehrlichiosis in an Australian cohort.
Int J Gen Med. 2015;8:15–26.
85. Middelveen MJ, Burugu D, Poruri A, et al. Association of spirochetal
infection with Morgellons disease. F1000Res. 2013;2:25.
86. Mayne P, English JS, Kilbane EJ, Burke JM, Middelveen MJ, Stricker
RB. Morgellons: a novel dermatological perspective as the multisystem
infective disease borreliosis. F1000Res. 2013;2:118.
87. Middelveen MJ, Bandoski C, Burke J, et al. Exploring the association between Morgellons disease and Lyme disease: identification of
Borrelia burgdorferi in Morgellons disease patients. BMC Dermatol.
2015;15:1.
88. Mursic VP, Wanner G, Reinhardt S, Wilske B, Busch U, Marget W.
Formation and cultivation of Borrelia burgdorferi spheroplast L-form
variants. Infection. 1996;24(3):218–226.
89. Brorson O, Brorson SH. An in vitro study of the susceptibility of mobile
and cystic forms of Borrelia burgdorferi to tinidazole. Int Microbiol.
2004;7(2):139–142.
90. Shah JS. Morgellons disease: chronic form of Borrelia infection?
Poster presented at: 9th Annual Medical Scientific Conference on
Morgellons Disease; April 30–May 1, 2016; Austin, TX.
91. Allen L, Saylor-Hefley C. Morgellons under investigation: identification of associated microorganisms by molecular analysis of epithelial
samples. Poster presented at: 7th Annual Medical Scientific Conference
on Morgellons Disease; March 29–30, 2014; Austin, TX.
92. Georgilis K, Peacock M, Klempner MS. Fibroblasts protect the Lyme
disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitro.
J Infect Dis. 1992;166(2):440–444.
93. Klempner MS, Noring R, Rogers RA. Invasion of human skin fibroblasts by the Lyme disease spirochete Borrelia burgdorferi. J Infect
Dis. 1993;167(5):1074–1081.
94. Chmielewski T, Tylewska-Wierzbanowska S. Interactions between
Borrelia burgdorferi and mouse fibroblasts. Pol J Microbiol.
2010;59(3):157–160.
95. Bandoski C. Evidence for the presence of human pathogens Borrelia
and Helicobacter in Morgellons patients’ skin samples. Poster presented at: 7th Annual Medical Scientific Conference on Morgellons
Disease; March 29–30, 2014; Austin, TX.
96. Middelveen MJ, Rotaru GM, McMurray JL, et al. Canine filamentous
dermatitis associated with Borrelia infection. J Vet Sci Med Diagn.
2016;5(6):1000212.
97. Malane MS, Grant-Kels JM, Feder HM Jr, Luger SW. Diagnosis of
Lyme disease based on dermatologic manifestations. Ann Intern Med.
1991;114:490–498.
98. Vrethem M, Hellblom L, Widlund M, et al. Chronic symptoms are
common in patients with neuroborreliosis: a questionnaire follow-up
study. Acta Neurol Scand. 2002;106(4):205–208.
99. Fallon BA, Schwartzberg M, Bransfield R, et al. Late-stage neuropsychiatric Lyme borreliosis: differential diagnosis and treatment.
Psychosomatics. 1995;36(3):295–300.
100. Gao HM, Hong JS. Why neurodegenerative diseases are progressive:
uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365.
101. Bransfield RC. The psychoimmunology of Lyme/tick-borne diseases
and its association with neuropsychiatric symptoms. Open Neurol J.
2012;6:88–93.
102. Kristensson K. Microbes’ roadmap to neurons. Nat Rev Neurosci.
2011;12(6):345–357.
103. Mattingley DW, Koola MM. Association between Lyme disease and
schizoaffective disorder, bipolar type: is it inflammation mediated?
Indian J Psychol Med. 2015;37(2):243–246.
104. Lange R, Seyyedi S. Evidence of a Lyme borreliosis infection
from the viewpoint of laboratory medicine. Int J Med Microbiol.
2002;291(33):120–124.
105. Schmidt BL. PCR in laboratory diagnosis of human Borrelia burgdorferi infections. Clin Microbiol Rev. 1997;10(1):185–201.
106. US Centers for Disease Control and Prevention. Lyme disease: diagnosis and testing. 2015. Available from: https://www.cdc.gov/lyme/
diagnosistesting/index.html. Accessed September 30, 2017.
107. Stricker RB, Johnson L. Serologic tests for Lyme disease: more smoke
and mirrors. Clin Infect Dis. 2008;47(8):1111–1112.
108. Cook MJ, Puri BK. Commercial test kits for detection of Lyme borreliosis: a meta-analysis of test accuracy. Int J Gen Med. 2016;9:427–440.
109. Dattwyler RJ, Volkman DJ, Luft BJ, Halperin JJ, Thomas J, Golightly
MG. Seronegative Lyme disease. N Engl J Med. 1988;319(22):
1441–1446.
110. Girard YA, Federova N, Lane RS. Genetic diversity of Borrelia
burgdorferi and detection of B. bissettii-like DNA in serum of north
coastal California residents. J Clin Microbiol. 2011;49(3):945–954.
111. Ogden NH, Margos G, Aanensen DM, et al. Investigation of genotypes
of Borrelia burgdorferi in Ixodes scapularis ticks collected during surveillance in Canada. Appl Environ Microbiol. 2011;77(10):3244–3254.
112. Péter O, Bretz AG, Bee D. Occurrence of different genospecies of
Borrelia burgdorferi sensu lato in Ixodid ticks of Valais, Switzerland.
Eur J Epidemiol. 1995;11(4):463–467.
113. Wilson ML, Weinstein MP, Barth Reller. Laboratory detection of
bacteremia and fungemia. In: Jorgenson JH, Pfaller MA, Carroll KC,
et al, editors. Manual of Clinical Microbiology. 11th ed. Washington:
ASM Press; 2015:15–29.
114. Söderfeldt Y, Gross D. Information, consent and treatment of patients
with Morgellons disease: an ethical perspective. Am J Clin Dermatol.
2014;15(2):71–76.
115. Lepping P, Russell I, Freudenmann RW. Antipsychotic treatment of
primary delusional parasitosis: systematic review. Br J Psychiatry.
2007;191:198–205.
116. Sultana A, McMonagle T. Pimozide for schizophrenia or related psychoses. Cochrane Database Syst Rev 2000;(2):CD001949.
117. Webster JP, Lamberton PH, Donnelly CA, Torrey EF. Parasites
as causative agents of human affective disorders? The impact of
anti-psychotic, mood-stabilizer and anti-parasite medication on
Toxoplasma gondii’s ability to alter host behaviour. Proc Biol Sci
2006;273(1589):1023–1030.
118. Hamann K, Avnstorp C. Delusions of infestation treated by pimozide: a double-blind crossover clinical study. Acta Derm Venereol.
1982;62(1):55–58.
119. Lindskov R, Baadsgaard O. Delusions of infestation treated with pimozide: a follow-up study. Acta Derm Venereol. 1985;65(3):267–270.
120. Eccles JA, Garfinkel SN, Harrison NA, et al. Sensations of skin infestation linked to abnormal frontolimbic brain reactivity and differences
in self-representation. Neuropsychologia. 2015;77:90–6.
121. Hirjak D, Huber M, Kirchler E, et al. Cortical features of distinct
developmental trajectories in patients with delusional infestation. Prog
Neuropsychopharmacol Biol Psychiatry. 2017;76:72–79.
122. Huber M, Wolf RC, Lepping P, et al. Regional gray matter volume
and structural network strength in somatic vs. non-somatic delusional
disorders. Prog Neuropsychopharmacol Biol Psychiatry. Epub 2017
Nov 24.
123. Livengood JA, Gilmore RD Jr. Invasion of human neuronal and glial
cells by an infectious strain of Borrelia burgdorferi. Microbes Infect.
2006;8(14–15):2832–2840.
124. Fallon BA, Levin ES, Schweitzer PJ, Hardesty D. Inflammation and
central nervous system Lyme disease. Neurobiol Dis. 2010;37(3):
534–541.


 Views Today : 71
Views Today : 71